A Primer on Aging: What if we could rejuvenate our cells? And how would it impact our aging population?
What is the future of cellular rejuvenation? Can we turn back the biological clock? Does targeting ageing make economic sense for payers, providers and investors? In this article, Anica Oesterle, board member with the Stanford Biotech Group, and an MS/MBA Student at Stanford, argues that the future of cellular rejuvenation is exciting. She describes the biology & research landscape for ageing & cellular rejuvenation, and assesses the economic implications of targeting ageing.
Anica Oesterle, MS/MBA Student, Stanford Graduate School of Business
Special thanks to Oliver Hahn and Janine Sengstack.
A Primer on Aging: What if we could rejuvenate our cells? And how would it impact our aging population?
If you are interested in stem cell research, you may have heard of scientists rejuvenating old mice by transiently activating four stem cell genes - the Nobel Prize-winning Yamanaka Factors. This is called partial reprogramming. If your realm is the tech world, you likely have heard of tech entrepreneurs making large bets on (and investments in) anti-aging start-ups. According to a recent MIT Technology Review article, even the kingdom of Saudi Arabia plans to spend up to $1 billion per year on aging research. Media are quick to jump to headlines like “Bezos’ bet on living forever”, fuelling the human desire to defeat aging that has been as old as time. Just look at Lucas Cranach’s 1546 Renaissance painting “The Fountain of Youth'', Mary Shelly’s famous Frankenstein or the 1992 movie “Death Becomes Her”, starring Meryl Streep and Goldie Hawn on a quest to defeat death. However, today’s cutting edge anti-aging research has little to do with “defeating death” (extending lifespan), but focuses on extending healthspan to find ways how we can live healthier longer. The company Altos Labs, for example, attempts to achieve this with “cellular rejuvenation programming” - the process of turning back the clock on the age of your cells to make them behave like young cells again (for example, healthier mitochondria, less DNA damage, better protein recycling). If successful, these discoveries will have a tremendous impact on humans in a much more concrete and realistic way than the SciFi vision of living forever.
According to the WHO, by 2030, one in six people will be aged 60 years and over. This equates to 1.4 billion people globally. By 2050, this number will almost double to 2.1 billion. The number of 80 year olds will even triple, to more than 425 million. These changes will further drive demographic change, while our healthcare systems will face a Herculean task. As we age, the risk of suffering from chronic illnesses is soaring. Age is the main risk factor for the most common diseases of developed countries: cancer, cardiovascular diseases, autoimmune diseases, and neurodegeneration (Niccoli et al., 2012). William Haseltine, a professor at Harvard Medical School, underlines that in view of this shift, countries will have to reconsider all aspects of their communities, “from healthcare systems and methods of delivering care to how whole cities are structured.” He suggests new cost effective approaches to elderly and long-term care that can meet the needs of this growing population group, while preventing skyrocketing costs. These approaches focus on a revision of the current care infrastructure, revisiting policy agendas, and reviewing healthcare financing systems. But what if we could “solve” aging at a genetic level? Researchers and professors such as Michael Snyder and Tony Wyss-Coray here at Stanford, Linda Partridge at University College London, and David Sinclair at Harvard Medical School study the biology and genetics of aging with the goal to extend healthy lifespan, which means: how can we live healthier longer? Let’s first investigate how we age on a cellular level to better understand the potential of this research for society as a whole.
The Biology of Aging: How Old are Your Cells?
Our chronological age is rarely aligned with our cellular age. While chronological age is determined by the time that has passed since we were born (i.e., “You are 27 years old”), cellular age is determined by the damage of a wide variety of molecular and cellular factors. In 2013, Carlos López-Otín and other researchers identified the so-called “hallmarks of aging” that are contributing to the aging process in our cells:
Genomic instability. Every day our cells are exposed to external factors that can damage our DNA - for example, cigarette smoke, sunlight (UV radiation), or industrial chemicals in our food or the environment. Our body has developed a complex network of genome maintenance systems to remove this damage. However, sometimes these systems make mistakes and don’t correctly “copy over” our genome into new cells. These mistakes can eventually lead to genetic mutations, which could cause diseases (Vijg et al., 2013).
Telomere attrition. Telomeres are the “caps” at the end of human chromosomes that ensure our DNA is copied correctly into new cells as cells divide. But after every cell division that copies the DNA into new cells, the telomeres get shorter. Hence, several scientists believe that older cellular age corresponds to shorter telomeres. Once the telomeres are too short to protect the DNA from damage, the cell stops dividing and turns into a “senescent” (old) cell (Artandi SE, 2022). However, there is conflict in the field on how much telomeres actually matter.
Epigenetic alterations. “Epigenetics” refers to DNA structure modifications that affect which genes in a cell are turned on or off, without modifications to the DNA sequence. We observe many of these epigenetic changes in our cells that can contribute to cell aging and related diseases such as cancer, neurodegenerative and cardiovascular decline, and autoimmune disorders (Gonzalo, 2010).
Loss of proteostasis. Proteostasis ensures that proteins are folded correctly and generated at the right time and cellular location, among other functions. It also removes superfluous and misfolded proteins. Misfolded and aggregated proteins can contribute to diseases. However, as we age, the functionality of the proteostasis system declines (Hipp et al., 2019).
Deregulated nutrient-sensing. Being able to sense and respond to fluctuations in our environmental nutrient levels is a prerequisite for life. “Nutrient-sensing” is the internal process by which our body senses and stores nutrients and energy when food is abundant, and draws from this internal nutrient storage when food is scarce. This nutrient sensing function is commonly deregulated in metabolic diseases (Efeyan et al., 2015). Studies found that inhibiting these nutrient-sensing “signaling pathways”, for example in the form of intermittent fasting or other forms of calorie restriction, can be associated with an extension in lifespan (Giacomello et al., 2021).
Mitochondrial dysfunction. Mitochondria are the powerhouses of our cells. They produce the energy carrier adenosine triphosphate (ATP) and have their own mitochondrial DNA. As we age, this mitochondrial DNA is increasingly prone to mutations and damage, which has been associated with mitochondrial dysfunction (Payne et al., 2015).
Cellular senescence. As soon as a cell that usually divides suddenly stops dividing forever it has reached “cellular senescence”. This can be triggered by various aging-associated stimuli. While cellular senescence is helpful in a young body because it prevents damaged cells from dividing and spreading further, it can be harmful in an old body. Damaged cells are no longer removed effectively, can accumulate and consequently, can harm the function of the wider tissue and increase inflammation in the cell (López-Otín et al., 2013).
Stem cell exhaustion. Multiple types of cellular damage that accumulate as we age also leave their mark on our stem cells - the cells from which all other cells with specialized functions are generated (Mayo Clinic). This “exhaustion” of our stem cells, meaning they no longer function properly, is directly responsible for many physical problems we experience at older age, such as a weaker immune system (López-Otín et al., 2013).
Altered intercellular communication. With increasing age our cells’ ability to communicate with each other declines. Intercellular communication is important for cells to grow and function normally. Cells that lose the ability to respond to signals from other cells may become cancer cells (National Cancer Institute).
Cellular Reprogramming: Can We Turn Back the Clock?
The door to epigenetic reprogramming was opened in 2006 by Dr. Shinya Yamanaka, a stem-cell researcher at Kyoto University who shared the 2012 Nobel Prize in medicine for his work. Yamanaka discovered four transcription factors - agents that activate genes - that could reverse a cell’s age and turn a differentiated cell back in time, into a stem cell. When the Yamanaka factors are targeted in a specific cell, this cell loses its identity and reverts back to its young, embryonic state (“induced pluripotent stem cell”). This “rejuvenation” process also erases several damaging aging phenomena the cell has accumulated over its lifetime. Scientists then wondered whether these stem cell genes could be used to turn back the aging clock a little bit. Not all the way back to stem cells, just to younger cells. Scientists turned on these four genes transiently in old mice, and the mice were indeed rejuvenated. But there is a serious clinical challenge. When these genes are turned on for too long in a mouse, the mouse’s cells de-differentiate into stem cells, which grow too quickly and cause cancer. Since then, multiple researchers and companies have been looking for ways to rejuvenate cells in a safer way to not only tackle the symptoms of diseases, but reverse the effects of aging-related diseases at their core - a discovery that would be a game changer for the future of human health.
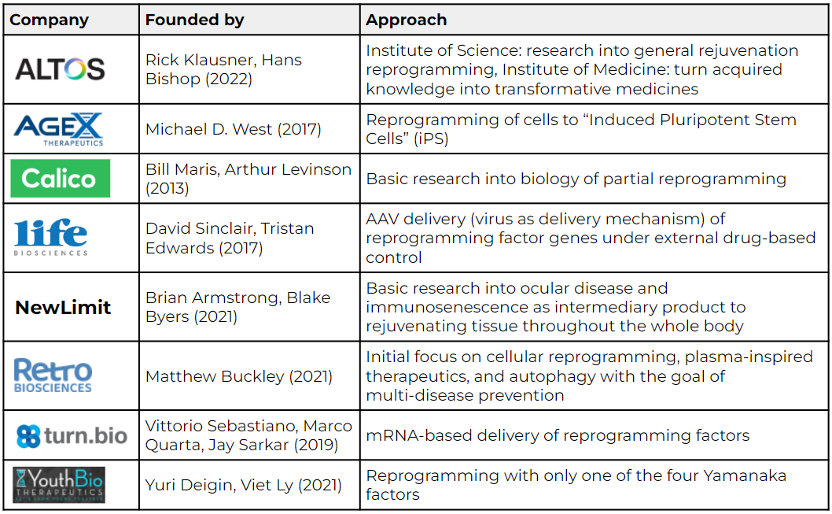
The following table highlights a few of the many companies that work on genetic reprogramming to rejuvenate our cells:
Sources: Eisenstein (Nature, 2022); AgeX, NewLimit, Retro Biosciences, and Altos Labs websites.
Altos Labs has received special attention since its official launch earlier this year due to its team of high-profile scientists, clinicians, industry leaders, and investors. Rick Klausner was former director of the National Cancer Institute and entrepreneur, while Hans Bishop was former CEO of GRAIL and Juno Therapeutics. Hal Barron, former Chief Science Officer at GSK, joined as CEO. While Altos’s exact approach is not public yet, the research community has high hopes for the well-funded start-up. Reversing cellular aging via genetic reprogramming or eventually other approaches is a comparably new research field with many unsolved complexities and unanswered questions. Start-ups in this space embark on a marathon, not a sprint, and need to be prepared for a long-term investment, according to Michael Eisenstein’s recent Nature article (2022). Altos Labs seems well equipped for the long race.
Using Artificial Intelligence to Identify Target Genes
As in many other fields, artificial intelligence can be helpful in cellular rejuvenation research. For example, the company Shift Bio is using machine learning to identify genes that help reverse cellular aging without turning cells back into their embryonic state and hence, avoid causing cancer. According to the company’s website, Shift is using public and proprietary gene expression data from cell reprogramming studies that are fed into its ML algorithms, the “Shift DC1 driver clock”, to identify the contributions different genes make to the rejuvenation process. Based on the gene expression data, Shift performs a dimensionality reduction from genes to the specific actions among molecules in a cell that are relevant to the cell aging process (“pathways”). Practically, this means that the number of input variables are reduced to the ones that matter most, while the others are discarded. In a second step, these pathways in the form of variables are used as input for Shift’s machine learning model. The model identifies the pathways that are related to the age-related changes and preserves information about the contribution of individual genes to the process (Shift Bioscience, 2021).
However, while researchers are dashing ahead with the vision and mission to “hack” how we age and transform all of human health, how is the regulatory system in the US keeping up?
“It is Time to Classify Biological Aging as a Disease” (Bulterijs et al., 2015)
Historically, the process of aging itself has been considered a natural and universal process (similar to development) that each human is undergoing and hence, not a disease. In contrast, several medical issues that are the consequence of aging, such as osteoporosis, hypertension, dementia, and diabetes, have been classified as diseases and accepted as targets for medical treatments (Bulterijs et al., 2015). Well, why do we care about whether aging is officially classified as a disease?
First, recognizing aging as a disease is essential for cellular rejuvenation therapies to be refunded by health insurance providers (Reznek, 1987). Second, aging under the disease label would shift cellular rejuvenation and other anti-aging therapies from the Federal Drug Administration’s (FDA) regulations for cosmetic medicine to stricter regulations for disease treatment and prevention (Gems, 2011). Research institutes would also be able to attract more outside funding to finance their resource-intensive aging research. Overall, this shift would ideally give rise to safe, effective and affordable genetic reprogramming therapies to reverse aging-related diseases in our aging population.
We know that aging research and cellular rejuvenation therapies are extremely promising for society as a whole to live healthier longer and may be able to greatly reduce the financial burden on our healthcare system. But let’s zoom in on the numbers. Does it pay off to target aging, not only for patients, but also for payors, providers, and investors?
Does it (Literally) Pay Off to Target Aging?
In 2021, Andrew Scott, Martin Ellison - economics professors at London Business School and Oxford University, respectively - as well as Harvard genetics professor David Sinclair did the math. Was it better to make lives healthier by reducing morbidity or longer by extending life? The team evaluated participants’ willingness to pay for increases in life expectancy, health improvements, and treatments that target aging (Scott et al., 2021). The result was eye opening: Patients value healthy years over simply more years of life. A health intervention that increased life expectancy by one year was worth $118,000 to the average American, but an additional year of healthy life was worth $242,000. People prefer aging well over living longer!
While the case is overwhelmingly positive for patients, innovation in healthcare is difficult because the service recipient (patient) is not paying the healthcare provider directly. Health insurance companies, as the intermediary, need to be incentivized as well. The value proposition of reversing diseases at their core with cellular rejuvenation reprogramming could pay off for insurance companies, too. Given age is a key factor in many diseases, slowing or reversing cellular aging “kills multiple birds with one stone” and offers cumulative benefits to patients with just one treatment. This may reduce overall costs for health insurance companies in the short-term already. There are also long-term benefits. In an article for a16z, Andrew Scott points out that targeting aging instead of individual diseases can unlock health synergies. Cellular rejuvenation therapies in a specific patient could both prevent cancer today, as well as dementia and osteoporosis tomorrow and consequently lower long-term costs for health insurance companies. A cellular rejuvenation therapy can be considered an upfront investment in a patient’s long-term health. Lastly, patients live healthier longer and hence, healthcare funds can be allocated to the patients with severe diseases, who really need medical care.
The success and failures of the pioneering companies in cellular rejuvenation research over the next decade will be critical not only for the US, but also for other countries with rapidly aging populations such as China, India, and Japan. I am excited to see these companies push the boundaries over the next years and decades!
References:
Ahadi S, Zhou W, Schüssler-Fiorenza Rose SM, Reza Sailani M, Contrepois K, Avina M, Ashland M, Brunet A, Snyder M. Personal aging markers and ageotypes revealed by deep longitudinal profiling. Nature, 2020. [Link]
Ann Ran F, Hsu PD, Wright J, Agarwala V, Scott D, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nature, 2013. [Link]
Armitage H. ‘Ageotypes’ provide window into how individuals age, Stanford study reports. Stanford Medicine, 2020. [Link]
Artandi SE. Telomeres in aging: small differences making a big phenotypic impact. Stanford University GENE 229 lecture, Winter Quarter 201/22.
Bulterijs S, Hull RS, Bjoerk VCE, Roy AG. It is time to classify biological aging as a disease. Frontiers in Genetics, 2015. [Link]
Caobi A, Kumar Dutta R, Garbinski LD, Esteban-Lopez M, Ceyhan Y, Andre M, Manevski M, Raj Ojha C, Lapierre J, Tiwari S, Parira T, El-Hage N. The impact of CRISPR-Cas9 on age-related disorders: from pathology to therapy. Aging and Disease, 2020. [Link]
Cyranoski D. The potential effects of Japan’s stem-cell policies. Nature, 2019. [Link]
Efeyan A, Comb WC, Sabatini DM. Nutrient sensing mechanisms and pathways. HHS Author Manuscript, 2015. [Link]
Eisenstein M. Rejuvenation by controlled reprogramming is the latest gambit in anti-aging. Nature, 2022. [Link]
Gems D. Tragedy and delight: the ethics of decelerated ageing. Philos Trans R Soc Lond B Biol Sci., 2011. [Link]
Giacomello E, Toniolo L. The potential of calorie restriction on calorie restriction mimetics in delaying aging: focus on experimental models. Nutrients, 2021. [Link]
Gonzalo S. Epigenetic alterations in aging. Journal of Applied Physiology, 2010. [Link]
Haseltine WA. Aging populations will challenge healthcare systems all over the world. Forbes, 2018. [Link]
Hipp MS, Kasturi P, Hartl FU. The proteostasis network and its decline in ageing. Nature, 2019. [Link]
Hu L, Zhao B, Wang S. Stem-cell therapy advances in China. Human Gene Therapy, 2018. [Link]
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The Hallmarks of Aging. NCBI, 2013. [Link]
MIT Technology Review. Saudi Arabia plans to spend $1 billion a year discovering treatments to slow aging. 2022. [Link]
National Human Genome Research Institute. Genetics Glossary, 2022. [Link]
Niccoli T, Partridge L. Ageing as a risk factor for disease. Current Biology, 2012. [Link]
Payne BAI, Chinnery PF. Mitochondrial dysfunction in aging: much progress but many unresolved questions. BBA Bioenergetics, 2015. [Link]
Reuters Plus. Intelligent regeneration in Japan. [Link]
Reznek L. The Nature of Disease. London: Routledge & Kegan Paul, 1987. [Link]
Scott AJ, Ellison M, Sinclair DA. The economic value of targeting aging. Nature, 2021. [Link]
Scott AJ. The average American would pay $242,000 for one extra year of good health. a16z Future, 2021. [Link]
Vijg J, Suh Y. Genome instability and aging. Annual Review of Physiology, 2013. [Link]
World Health Organization. Ageing and health. WHO Newsroom, 2021. [Link]
About the author
Anica Oesterle
Anica is a MS/MBA student at Stanford School of Engineering and Graduate School of Business. After three years in Goldman Sachs’ Investment Banking team across London and San Francisco, Anica joined the German Initiative for Artificial Intelligence, appliedAI, as an AI Engineer, where she discovered her interest for applications of Machine Learning in biotech. Outside of work and academia, Anica’s main interests are architecture, literature & philosophy, coffee, and boxing classes.